Stroke Outcomes
The charts below showcase recent outcomes for patients receiving acute stroke treatments at Mount Carmel East's Comprehensive Stroke Center.
| Acute Ischemic Stroke Intervention Outcomes* | 24 Hours Post-Intervention Outcomes |
|---|---|
| Patients treated for acute ischemic stroke at Mount Carmel East showed clinical improvement within 24 hours (based on pre- and post-NIHSS) |
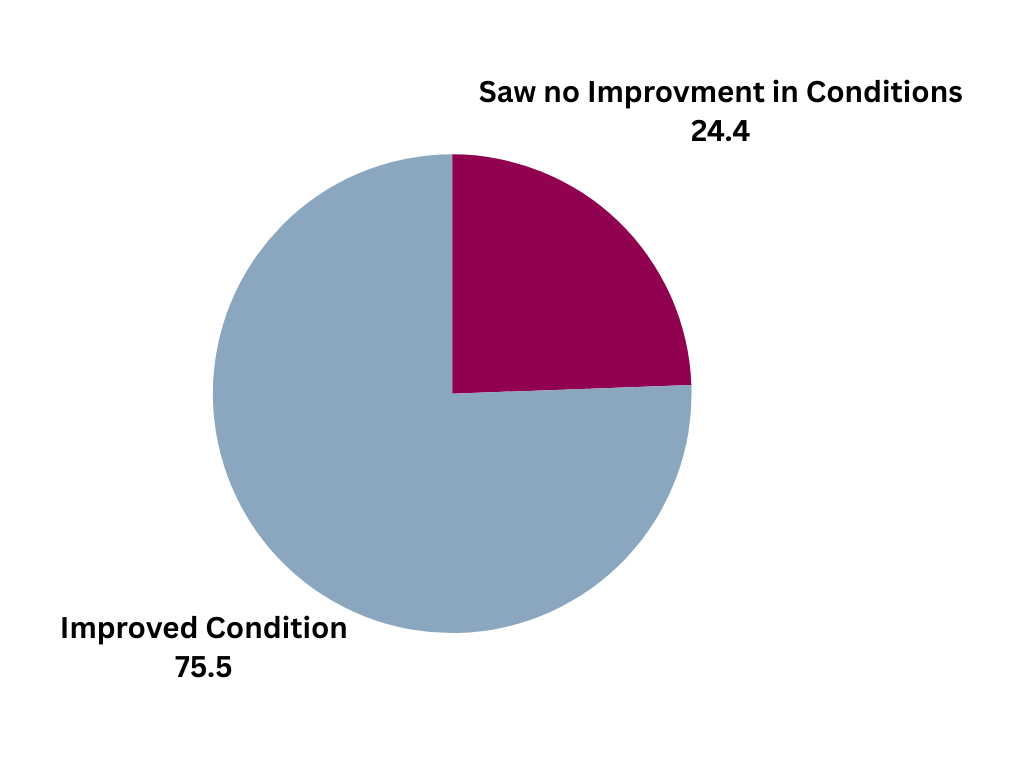 |
*Includes all patients who received intravenous and/or intra-arterial thrombolytic therapy ("r-tPA, Activase/Alteplase, etc.) and/or mechanical endovascular reperfusion therapy ("clot retrieval", "MERT", etc. at Mount Carmel East between January 2022 and December 2023.
| Acute Ischemic Stroke Intervention Outcomes* | Post-Intervention Discharge Outcomes |
|---|---|
| Patients treated for acute ischemic stroke at Mount Carmel East showed clinical improvement by hospital discharge (based on pre- and post-NIHSS) |
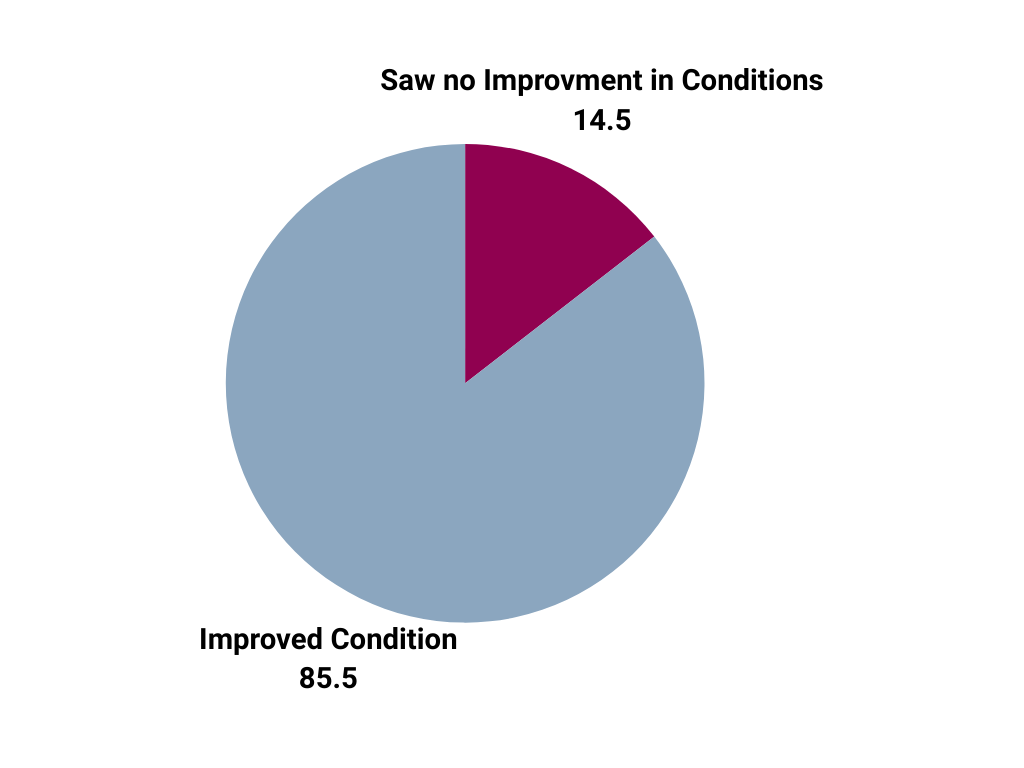 |
*Includes all patients who received intravenous and/or intra-arterial thrombolytic therapy ("r-tPA, Activase/Alteplase, etc.) and/or mechanical endovascular reperfusion therapy ("clot retrieval", "MERT", etc. at Mount Carmel East between January 2022 and December 2023.
| Acute Ischemic Stroke Treatment Bleeding Complications | < 36 hour Post-Treatment Hemorrhagic Complication Rate |
|---|---|
| When compared to other certified Comprehensive Stroke Centers, Mount Carmel East's hemorrhagic complication rate (2.39%)* for patients who received an acute ischemic stroke treatment is considerably lower than the national average (5.4%).** |
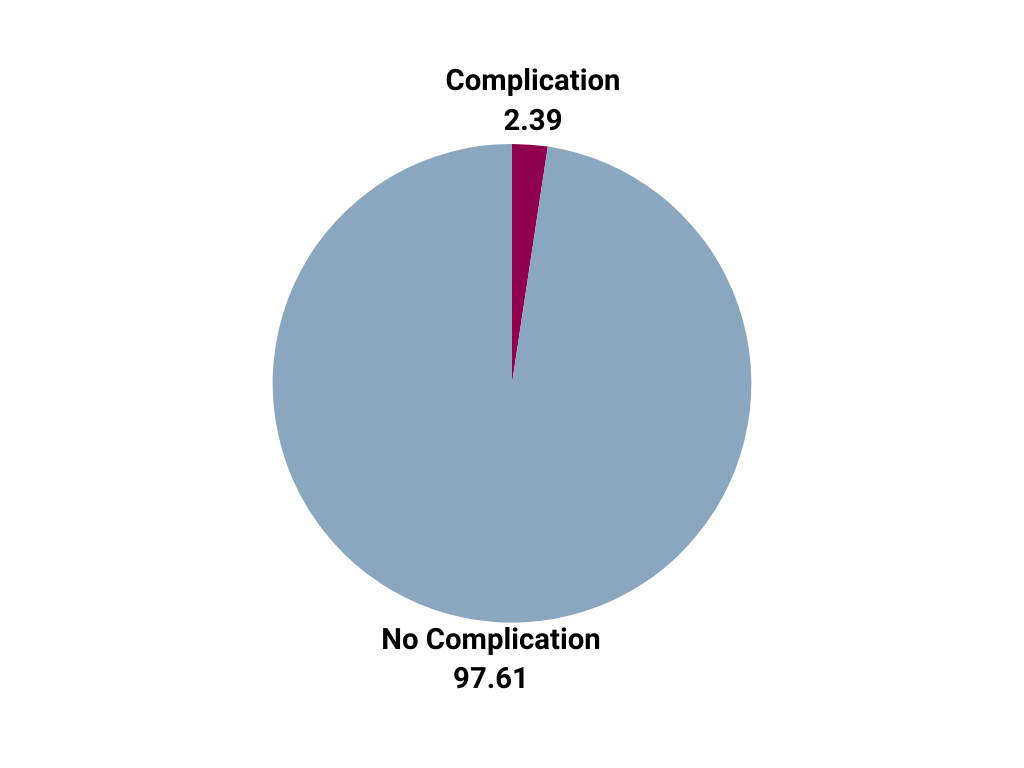 |
*Per The Joint Commission's CSTK-5 Measure Definition: Ischemic stroke patients who develop a symptomatic intracranial hemorrhage (i.e., clinical deterioration ≥ 4 point increase on NIHSS and brain image finding of parenchymal hematoma, or subarachnoid hemorrhage, or intraventricular hemorrhage) within (≤) 36 hours after the onset of treatment with intra-venous (IV) or intra-arterial (IA) thrombolytic (t-PA) therapy, or mechanical endovascular reperfusion procedure (i.e., mechanical endovascular thrombectomy with a clot retrieval device).
**Includes all Joint Commission certified Comprehensive Stroke Centers who submitted data to Get with the Guidelines/American Stroke Association between January 1, 2022 and December 31, 2023.
